Site Blog
Recent Blog
contact us
- If you have questions, please contact us, all questions will be answered
- Email : David@tmaxcn.com
- Email : Davidtmaxcn@gmail.com
- Add : No. 39, Xinchang Road, Xinyang, Haicang Dist., Xiamen, Fujian, China (Mainland)
hot products
Blog
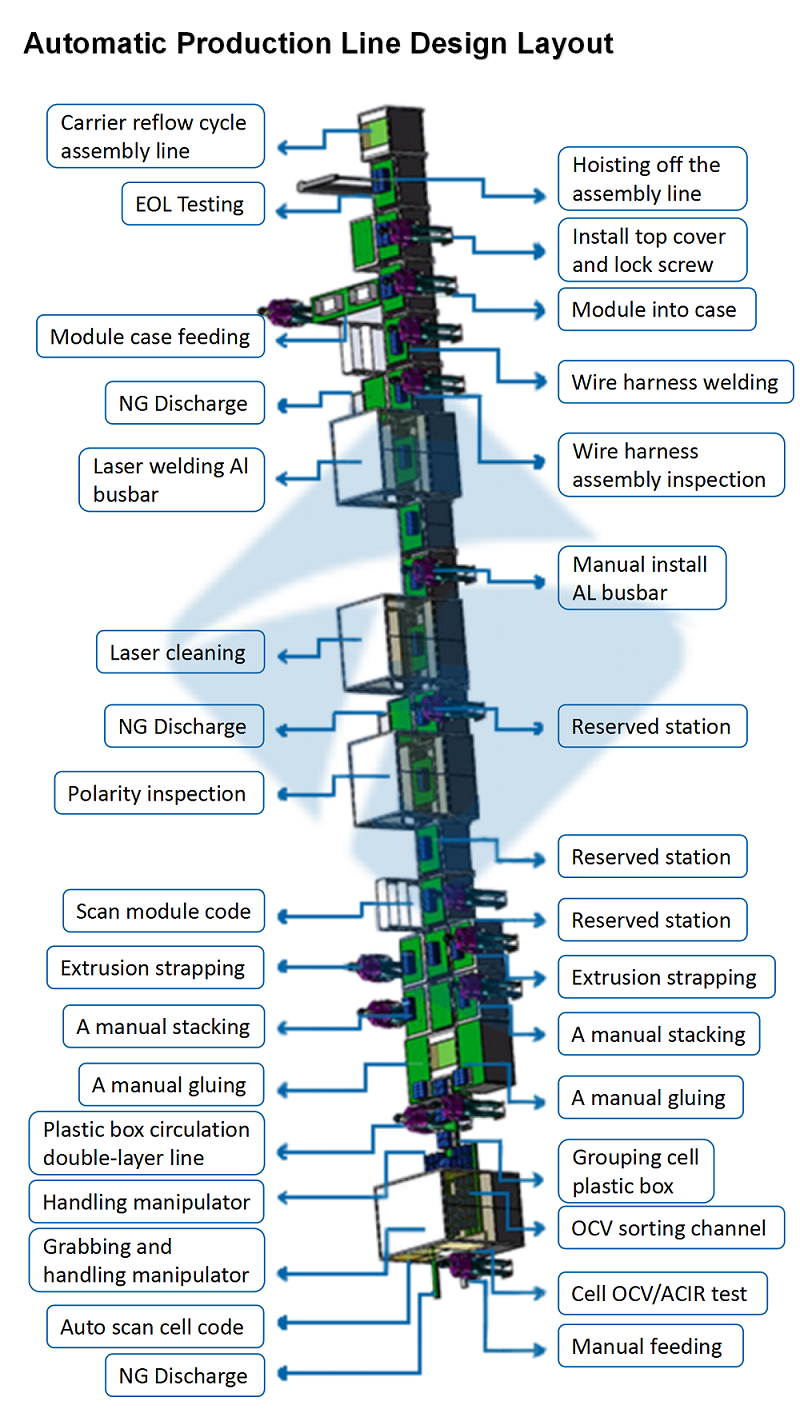
Cell Manufacturing Line
September 19,2024.
Cell Manufacturing Line: The Core of Battery Production
Cell manufacturing line is the backbone of battery production, responsible for assembling the individual battery cells that are used in a wide range of applications, from electric vehicles (EVs) and consumer electronics to energy storage systems. The efficiency, precision, and quality control within the cell manufacturing process directly affect the performance, safety, and longevity of the final battery.
In this article, we will explore the key stages of a typical Pouch Cell Manufacturing Line, the technologies used, and how automation is driving advancements in battery production.
●What is a Cell Manufacturing Line?
Cell manufacturing line consists of a series of integrated processes that transform raw materials (such as electrode materials, electrolytes, and separators) into fully assembled battery cells. These cells can be in different formats, including cylindrical, prismatic, or pouch cells, depending on the intended application.
Each step in the Lithium Cell Manufacturing Line is critical to ensuring that the cells meet the required specifications in terms of energy density, cycle life, and safety.
●Key Stages of the Cell Manufacturing Line
The cell manufacturing process can be broadly divided into several key stages:
1.Electrode Production
The cell assembly process begins with the production of electrodes—both anode and cathode—through a series of steps includingmixing,coating,drying, andcalendering.
-Mixing: Active materials (e.g., Lithium-based compounds for the cathode, graphite or silicon for the anode), binders, conductive agents, and solvents are mixed to create a uniform slurry.
-Coating: The slurry is coated onto a current collector (usually aluminum foil for cathodes and copper foil for anodes) using anelectrode coating machine.
-Drying: The coated electrode is passed through a drying oven to evaporate the solvent, leaving behind a solid film of active material.
-Calendering: The dried electrode is compressed using rollers to achieve the desired thickness and density, which is essential for improving the electrochemical performance of the battery.
2.Electrode Cutting and Slitting
After the electrodes are produced, they are cut into the appropriate sizes and shapes based on the design of the battery cell. In large-scale production, precision cutting tools ensure that each electrode is identical, which is critical for maintaining consistency across the battery cells.
-Slitting: In the case of cylindrical and prismatic cells, long electrode sheets are slit into narrow strips, while for pouch cells, the electrodes are cut into larger, flat sheets.
-Electrode Stacking: For prismatic and pouch cells, the electrodes are stacked layer by layer, alternating between anode, separator, and cathode materials.
3.Cell Assembly
Once the electrodes are cut and prepared, they are assembled into the desired cell format. The assembly process differs depending on the type of cell being manufactured:
-Cylindrical Cells: The electrodes are wound into a tight spiral (jelly-roll) configuration, with a separator in between the anode and cathode to prevent short circuits.
-Prismatic Cells: In these cells, the electrodes are stacked in layers and encased in a rigid metal shell, offering high energy density in a compact form.
-Pouch Cells: The electrodes and separator are stacked in a flat arrangement, and the cell is sealed in a flexible pouch, allowing for lightweight and high-energy applications.
4.Electrolyte Filling
Once the cells are assembled, the next crucial step iselectrolyte filling. The electrolyte is the medium through which lithium ions move between the anode and cathode during charge and discharge cycles. The most common electrolyte used in lithium-ion batteries is a lithium salt dissolved in a solvent.
-Vacuum Filling: Electrolyte filling is often done under vacuum conditions to ensure that the electrolyte penetrates all the layers of the electrode and separator, eliminating any air pockets that could lead to defects.
5.Sealing and Welding
After the electrolyte has been filled, the battery cell is sealed to prevent leakage and to maintain a controlled environment for the chemical reactions inside the cell. This is done differently for various cell types:
-Cylindrical Cells: The top and bottom of the cell are sealed with metal caps, often using laser or ultrasonic welding techniques.
-Prismatic Cells: The metal case is sealed using either laser welding or mechanical crimping.
-Pouch Cells: The pouch material is heat-sealed to form an airtight enclosure around the cell.
6.Formation and Aging
The assembled battery cells then undergo a process calledformation, where they are charged and discharged for the first time under controlled conditions. This step is essential for forming thesolid electrolyte interface (SEI) on the anode, which helps protect the active materials and improve battery performance.
-Formation Process: The cells are subjected to a controlled charging and discharging process to activate the electrochemical components and ensure stable performance.
-Aging: After formation, the cells are aged (stored) for a set period, allowing for any chemical reactions to stabilize. This step helps identify defective cells and ensures consistency in battery performance.
7.Final Testing and Grading
Once the formation and aging processes are complete, the cells undergo rigorous testing to ensure they meet the required specifications for capacity, voltage, internal resistance, and safety.
-Capacity Testing: Measures the actual energy storage capacity of the cell to ensure it aligns with design specifications.
-Voltage Testing: Ensures that the cell operates within the correct voltage range.
-Safety Testing: Includes tests for mechanical stability, short circuits, overcharging, and other safety concerns.
Cells that meet all the necessary criteria are graded according to their performance and can then be integrated into battery packs for end-use applications.
●Automation in Cell Manufacturing
The drive forautomation in cell manufacturing is a key trend in the battery industry. Automated cell production lines increasethroughput, improvequality control, and reducelabor costs, making them critical for mass production, especially in industries such as electric vehicles and energy storage systems.
Benefits of Automation:
1.Increased Precision: Automated systems ensure that each cell is produced with the same level of accuracy, reducing variability and defects.
2.Higher Throughput: Automation speeds up production, allowing for the manufacture of millions of cells annually.
3.Improved Quality Control: Automated inspection systems use sensors and machine learning algorithms to detect defects early in the process, minimizing waste.
4.Cost Efficiency: Automation reduces the need for manual labor, which lowers operational costs, especially in large-scale manufacturing plants.
●Challenges in Cell Manufacturing
Despite advances in automation and technology, cell manufacturing lines face several challenges:
-Material Handling: Sensitive materials like lithium must be handled with care to avoid contamination and degradation.
-Consistency: Achieving uniformity across large batches of cells is essential for ensuring long-term performance and safety, but variations in raw materials and processing conditions can affect quality.
-Scaling Up: As demand for batteries continues to grow, scaling up production while maintaining high quality and low costs remains a key challenge.
●Conclusion
Cell manufacturing line is a complex and highly integrated process that involves multiple stages, from electrode production and assembly to electrolyte filling and final testing. The precision and quality of each step are critical to the overall performance, safety, and reliability of the battery cells.
Cell manufacturing line is the backbone of battery production, responsible for assembling the individual battery cells that are used in a wide range of applications, from electric vehicles (EVs) and consumer electronics to energy storage systems. The efficiency, precision, and quality control within the cell manufacturing process directly affect the performance, safety, and longevity of the final battery.
In this article, we will explore the key stages of a typical Pouch Cell Manufacturing Line, the technologies used, and how automation is driving advancements in battery production.
●What is a Cell Manufacturing Line?
Cell manufacturing line consists of a series of integrated processes that transform raw materials (such as electrode materials, electrolytes, and separators) into fully assembled battery cells. These cells can be in different formats, including cylindrical, prismatic, or pouch cells, depending on the intended application.
Each step in the Lithium Cell Manufacturing Line is critical to ensuring that the cells meet the required specifications in terms of energy density, cycle life, and safety.
●Key Stages of the Cell Manufacturing Line
The cell manufacturing process can be broadly divided into several key stages:
1.Electrode Production
The cell assembly process begins with the production of electrodes—both anode and cathode—through a series of steps includingmixing,coating,drying, andcalendering.
-Mixing: Active materials (e.g., Lithium-based compounds for the cathode, graphite or silicon for the anode), binders, conductive agents, and solvents are mixed to create a uniform slurry.
-Coating: The slurry is coated onto a current collector (usually aluminum foil for cathodes and copper foil for anodes) using anelectrode coating machine.
-Drying: The coated electrode is passed through a drying oven to evaporate the solvent, leaving behind a solid film of active material.
-Calendering: The dried electrode is compressed using rollers to achieve the desired thickness and density, which is essential for improving the electrochemical performance of the battery.
2.Electrode Cutting and Slitting
After the electrodes are produced, they are cut into the appropriate sizes and shapes based on the design of the battery cell. In large-scale production, precision cutting tools ensure that each electrode is identical, which is critical for maintaining consistency across the battery cells.
-Slitting: In the case of cylindrical and prismatic cells, long electrode sheets are slit into narrow strips, while for pouch cells, the electrodes are cut into larger, flat sheets.
-Electrode Stacking: For prismatic and pouch cells, the electrodes are stacked layer by layer, alternating between anode, separator, and cathode materials.
3.Cell Assembly
Once the electrodes are cut and prepared, they are assembled into the desired cell format. The assembly process differs depending on the type of cell being manufactured:
-Cylindrical Cells: The electrodes are wound into a tight spiral (jelly-roll) configuration, with a separator in between the anode and cathode to prevent short circuits.
-Prismatic Cells: In these cells, the electrodes are stacked in layers and encased in a rigid metal shell, offering high energy density in a compact form.
-Pouch Cells: The electrodes and separator are stacked in a flat arrangement, and the cell is sealed in a flexible pouch, allowing for lightweight and high-energy applications.
4.Electrolyte Filling
Once the cells are assembled, the next crucial step iselectrolyte filling. The electrolyte is the medium through which lithium ions move between the anode and cathode during charge and discharge cycles. The most common electrolyte used in lithium-ion batteries is a lithium salt dissolved in a solvent.
-Vacuum Filling: Electrolyte filling is often done under vacuum conditions to ensure that the electrolyte penetrates all the layers of the electrode and separator, eliminating any air pockets that could lead to defects.
5.Sealing and Welding
After the electrolyte has been filled, the battery cell is sealed to prevent leakage and to maintain a controlled environment for the chemical reactions inside the cell. This is done differently for various cell types:
-Cylindrical Cells: The top and bottom of the cell are sealed with metal caps, often using laser or ultrasonic welding techniques.
-Prismatic Cells: The metal case is sealed using either laser welding or mechanical crimping.
-Pouch Cells: The pouch material is heat-sealed to form an airtight enclosure around the cell.
6.Formation and Aging
The assembled battery cells then undergo a process calledformation, where they are charged and discharged for the first time under controlled conditions. This step is essential for forming thesolid electrolyte interface (SEI) on the anode, which helps protect the active materials and improve battery performance.
-Formation Process: The cells are subjected to a controlled charging and discharging process to activate the electrochemical components and ensure stable performance.
-Aging: After formation, the cells are aged (stored) for a set period, allowing for any chemical reactions to stabilize. This step helps identify defective cells and ensures consistency in battery performance.
7.Final Testing and Grading
Once the formation and aging processes are complete, the cells undergo rigorous testing to ensure they meet the required specifications for capacity, voltage, internal resistance, and safety.
-Capacity Testing: Measures the actual energy storage capacity of the cell to ensure it aligns with design specifications.
-Voltage Testing: Ensures that the cell operates within the correct voltage range.
-Safety Testing: Includes tests for mechanical stability, short circuits, overcharging, and other safety concerns.
Cells that meet all the necessary criteria are graded according to their performance and can then be integrated into battery packs for end-use applications.
●Automation in Cell Manufacturing
The drive forautomation in cell manufacturing is a key trend in the battery industry. Automated cell production lines increasethroughput, improvequality control, and reducelabor costs, making them critical for mass production, especially in industries such as electric vehicles and energy storage systems.
Benefits of Automation:
1.Increased Precision: Automated systems ensure that each cell is produced with the same level of accuracy, reducing variability and defects.
2.Higher Throughput: Automation speeds up production, allowing for the manufacture of millions of cells annually.
3.Improved Quality Control: Automated inspection systems use sensors and machine learning algorithms to detect defects early in the process, minimizing waste.
4.Cost Efficiency: Automation reduces the need for manual labor, which lowers operational costs, especially in large-scale manufacturing plants.
●Challenges in Cell Manufacturing
Despite advances in automation and technology, cell manufacturing lines face several challenges:
-Material Handling: Sensitive materials like lithium must be handled with care to avoid contamination and degradation.
-Consistency: Achieving uniformity across large batches of cells is essential for ensuring long-term performance and safety, but variations in raw materials and processing conditions can affect quality.
-Scaling Up: As demand for batteries continues to grow, scaling up production while maintaining high quality and low costs remains a key challenge.
●Conclusion
Cell manufacturing line is a complex and highly integrated process that involves multiple stages, from electrode production and assembly to electrolyte filling and final testing. The precision and quality of each step are critical to the overall performance, safety, and reliability of the battery cells.
With the increasing demand for high-performance batteries in electric vehicles, consumer electronics, and grid storage, advancements in automation and technology are essential for scaling up production and meeting global needs. By optimizing the cell manufacturing line, battery manufacturers can produce more efficient, cost-effective, and reliable energy storage solutions.
 ru
ru
 Iris@tmaxcn.com
Iris@tmaxcn.com David@tmaxcn.com
David@tmaxcn.com +86 13174506016
+86 13174506016 18659217588
18659217588